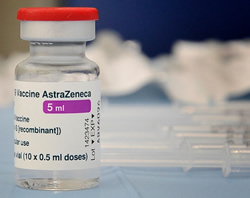
 The Australian Technical Advisory Group on Immunisation (ATAGI) has released a statement relating to AstraZeneca vaccinations to COVID-19 and a reported case of unusual thrombosis.
The Australian Technical Advisory Group on Immunisation (ATAGI) has released a statement relating to AstraZeneca vaccinations to COVID-19 and a reported case of unusual thrombosis.
ATAGI said the case followed an injection of the AstraZeneca vaccine, the clinical details of which were under review by the Therapeutic Goods Administration (TGA).
“ATAGI has not changed its advice on the use of the AstraZeneca vaccine at this time,” the Advisory Group said.
“This is one case in more than 400,000 doses given to date,” it said.
“We have issued communication for consumers and clinicians on the significance of this condition and to be alert for the symptoms and signs of thromboses.”
ATAGI said regulatory Agencies in the European Union and United Kingdom, where more cases had been reported and millions of doses of vaccine had been administered, hadn’t made recommendations to broadly restrict the use of the AstraZeneca vaccine.
“However, some countries, including Canada, have made precautionary recommendations to limit the use of AstraZeneca vaccine in some groups,” it said.
It said people who’d received COVID-19 vaccines should be aware of the common side effects which included fever, sore muscles, tiredness and headache.
ATAGI said the symptoms usually started within 24 hours of vaccination and lasted for one to two days.
“These side effects are expected and are not of concern unless severe or persistent,” it said.
“Consumers should be particularly alert to severe, persistent headaches that are different to their ‘usual’ pattern and do not settle with paracetamol or other painkillers.”
ATAGI said providers should also be aware of the warning signs of a severe condition associated with thrombosis and thrombocytopenia.
It said that if central venous sinus thrombosis (CVST), or another severe thrombotic complication, was suspected in a patient who’d received the AstraZeneca vaccine the patient should be referred to an emergency department for further assessment and haematology consultation.